Framework for regulatory compliance for software supplier in pharmaceutical manufacturing
While finishing my studies in Aalto University I faced a dilemma. How to combine Bachelors in Automation and System technology and Masters in Human Life Science technologies?
Luckily by taking a step back and looking into Medical and Pharmaceutical manufacturing provided the solution. That combined well with Prosys OPC need for a more documented Quality Management System (QMS). I could combine my multidisciplinary Automation background with Life tech and regulatory understanding and make something new.
To keep this test a recap, we will only scratch the surface on many topics. For full text please head to Aalto Archive after reading.
What is this Thesis about?
The pharmaceutical and medical industries are one of the most regulated industries on the planet and for a good reason. This regulation affects the products themselves, but also the manufacturing process and even the supply chain, also for a good reason. The regulations are complex and varied and must be understood by any stakeholders before they can contribute in the industry.
The issue is that the regulation is seen as a barrier of entry to contribute to the field. While the research is inconclusive, at least the mindset does exists and one of the effects is slowing down innovations and progress such as Pharma 4.0 adaptation.
As opportunity and challenge often go hand in hand, I started thinking: Would it be possible to ease some of this regulatory burden? Would it be possible for the suppliers take on some of the regulatory burden and benefit from it?
With this in mind, I set out to make a Framework that would assist a pharma/medical manufacturing software supplier to pass the regulatory hurdles, educate them about pharma thinking and help prepare for pharma needs. Essentially building path for a smaller companies to adopt Quality thinking, while not yet investing into costly certifications and high-level quality systems.
Background
Regulatory systems have evolved based on history of the region. For example, one reason for strict regulation in EU is the Thalidomide disaster in 1960. Similar events have caused areas such as USA, EU and Asia to have their own distinct regulatory philosophies. The regulations are being harmonized over time by ICH.
Regulations most likely most relevant to the reader are different Good Manufacturing Practices (GMP).
- EU GMP and MDR
- USA cGMP
- WHO GMP
- ISPE GAMP 5
- ISO Standards
These all set the regulatory and quality standard for the manufacturer of medical/pharmaceutical products. One of which is generally the supplier management and supplier audit. However, the guidelines for the suppliers range from vague to non existent. This brings us back to the question, how does one as a smaller company contribute to Pharma and more specifically pharma manufacturing?
Framework
My thesis answers the question in a form of a Framework.
The structured Framework is designed to assist software suppliers in navigating the complex regulatory landscape of pharmaceutical manufacturing. The aim is to streamline supplier compliance and contribution with GMP and QMS requirements, enabling development towards even safer and more efficient production of pharmaceuticals and medical devices.
In summary the Framework is built to provide easy steps to achieve a minimal QMS. It prepares the company to the pharma thinking model of “if it is not documented it does not exist” and prepares the company for upcoming audit. In addition, it provides the company basic education on regulation and a base from which to extend from in the future.
The framework is built from combining requirements and reoccurring themes from the earlier mentioned GMPs and Guidelines and turning them in to 4 parts with 13 easy to follow steps.
In a nutshell, the framework gives the user pre requirements to begin the QMS work. Then the user is directed to start the work with company assessments, preparations and creation of a Risk Plan. In addition, the user has to prepare documentation practices.
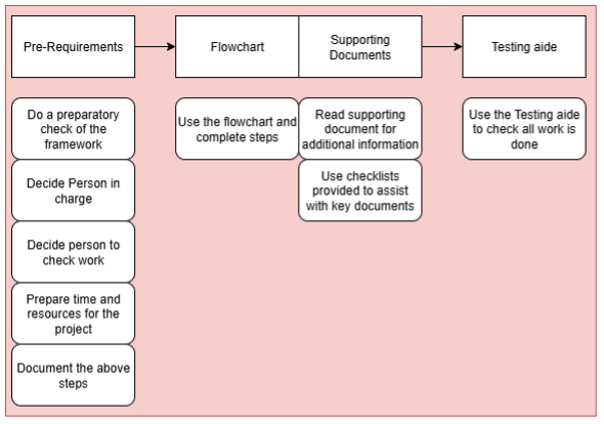
Fig 1: Pre requirements and use of the Framework
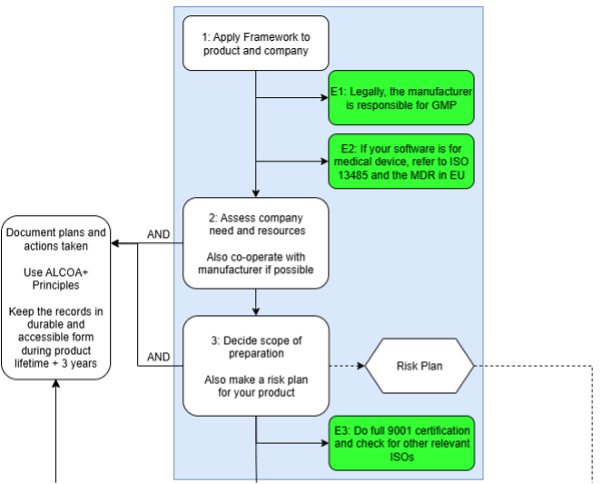
Fig 2: Preparatory part of the Framework
The main part of the work is to create and manage documentation and processes to provide quality evidence. Examples of creation part are making Standard Operating Procedures(SOP) for Software Lifecycle Management(SLCM) and Corrective And Preventative Actions(CAPA). In addition the framework user is directed to create required documentation, such as organizational charts, contact details and technical documentation.
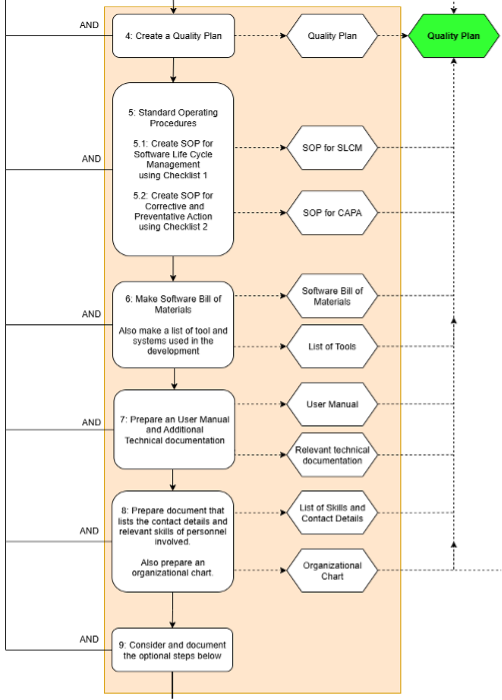
Fig 3: Creation part of the Framework
Final part of the framework is optional part, where the user can prepare further. This includes preparing for Audit or Agreement discussions or further working on Business Continuity Plan or Quality Risk Plan.
The final step is to define criteria for re-evaluation and improvement of the QMS and joining all the created documents in to a finalized quality plan. In addition, user is instructed on the requirement of constant document upkeep.
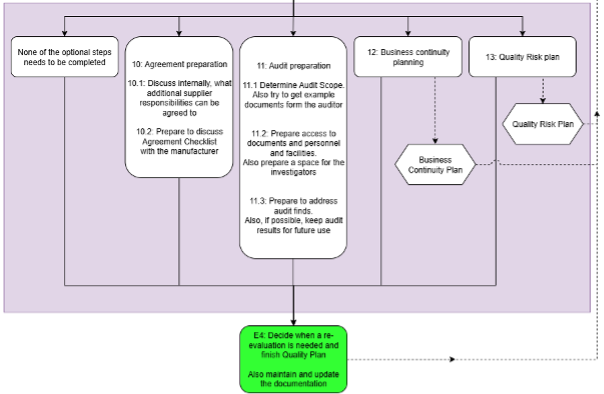
Fig 4: Optional part and end of the Framework
Case study: OPC UA Forge
After development the Framework was applied to OPC UA Forge and Prosys OPC as a company. The goal was to demonstrate the usability of the Framework and to prepare for an audit by an international pharmaceutical company.
Framework was followed step by step and relevant actions were taken. As result the company adapted a minimal QMS and the conducted audit went well.
The case was somewhat lucky for the writer as many processes and documentation already existed and the work was simply to document what was already done. However, some processes required additional steps or minor modification to produce additional Quality Evidence. Documentation practices were also developed further.
Conclusions
The results show that the framework improves regulatory readiness, supports audit success, and assists new stakeholders in contributing in the pharmaceutical industry.
Framework is an useful tool in creating QMS and a functional educational tool for learning about Pharma way thinking. However, it is not an official standard and does not contain template documents, thus the QMS will mirror the company making it. Also, pharmaceutical and medical field is vast, and thus the framework has hard time preparing the supplier of additional specific needs of some manufacturers.
If we take a step back and examine the framework in a larger context, the framework can be a useful tool at large even outside of Pharma. This is because it tries to bring out the best guidelines from regulation to aid in development and innovation. However, extra care is required by the user, as their regulations might have some specifics.
Further adaptation would also bring out missing items and show how the framework could be improved otherwise. Also, adaptation in start-up eco system could be a possibility as they generally work with small resources and fast pace.
The framework would benefit from comparisons to established QMS frameworks such as ISO 9001. The comparisons could focus on cost effectiveness, passed audits and similar metrics.
Final thoughts
The Framework was a useful tool in QMS creation, and it can enable new stakeholders and startups to enter the regulated pharmaceutical sector more confidently, contributing to global health and industry modernization.
However, further adaption would require some further development. Future developments could focus for example on integrating EU CRA requirements and expert opinions to the Framework. However, such development might eventually run into the one more standard issue.
If you want to hear more about Quality practices in Prosys OPC or the OPC UA Forge used in the case study, please don’t hesitate to contact us at sales@prosysopc.com.

Henri Toivola
Sales Engineer, Prosys OPC
Email: henri.toivola@prosysopc.com
